How four letters can change the liver metabolism
Objective: Farnesoid X receptor (FXR) plays a prominent role in hepatic lipid metabolism. The FXR gene encodes four proteins with structural differences suggestive of discrete biological functions about which little is known.
Methods: We expressed each FXR variant in primary hepatocytes and evaluated global gene expression, lipid profile, and metabolic fluxes. Gene delivery of FXR variants to Fxr(-/-) mouse liver was performed to evaluate their role in vivo. The effects of fasting and physical exercise on hepatic Fxr splicing were determined.
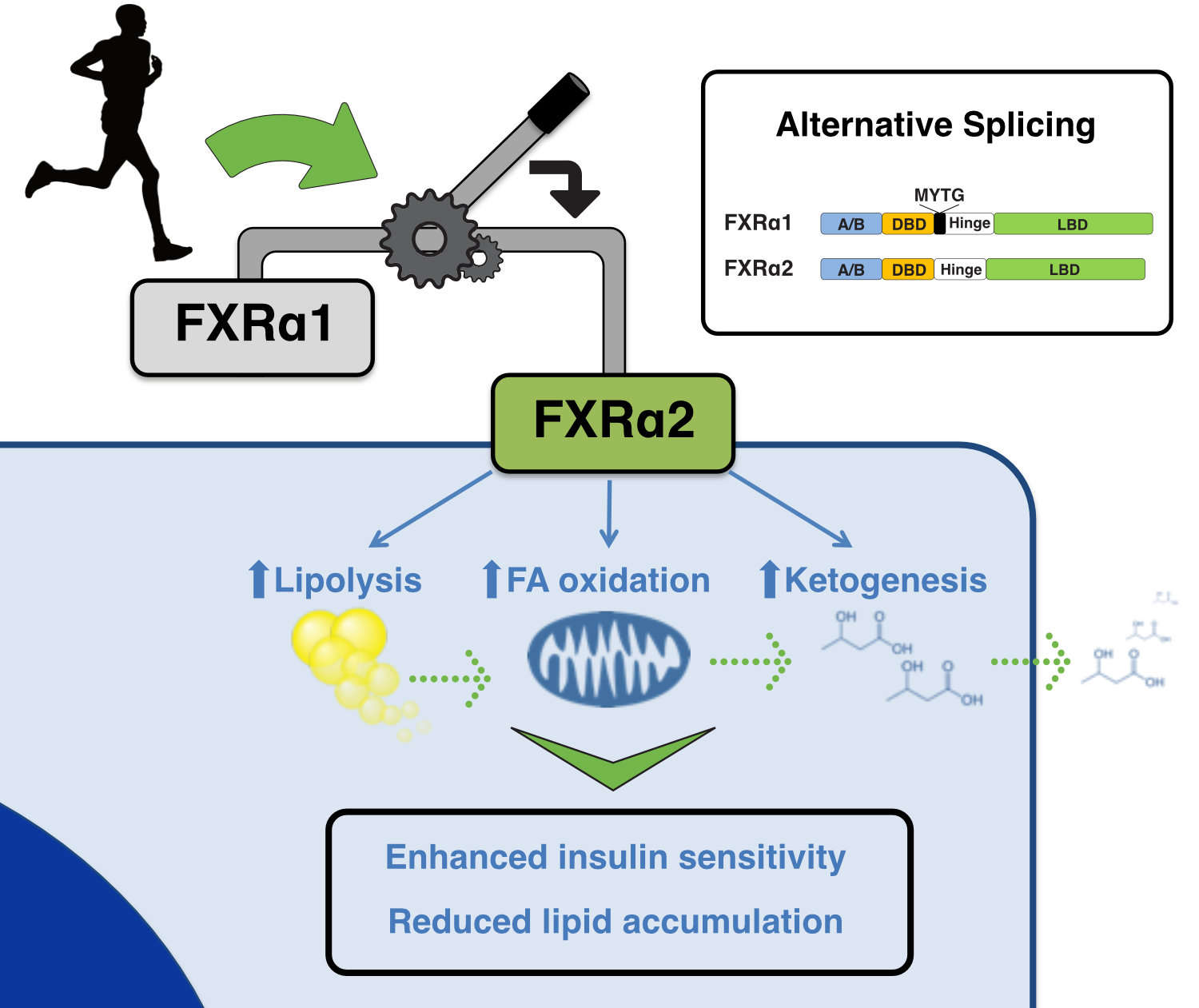
Results: We show that FXR splice isoforms regulate largely different gene sets and have specific effects on hepatic metabolism. FXRα2 (but not α1) activates a broad transcriptional program in hepatocytes conducive to lipolysis, fatty acid oxidation, and ketogenesis. Consequently, FXRα2 decreases cellular lipid accumulation and improves cellular insulin signaling to AKT. FXRα2 expression in Fxr(-/-) mouse liver activates a similar gene program and robustly decreases hepatic triglyceride levels. On the other hand, FXRα1 reduces hepatic triglyceride content to a lesser extent and does so through regulation of lipogenic gene expression. Bioenergetic cues, such as fasting and exercise, dynamically regulate Fxr splicing in mouse liver to increase Fxrα2 expression.
Conclusions: Our results show that the main FXR variants in human liver (α1 and α2) reduce hepatic lipid accumulation through distinct mechanisms and to different degrees. Taking this novel mechanism into account could greatly improve the pharmacological targeting and therapeutic efficacy of FXR agonists.
Bioenergetic cues shift FXR splicing towards FXRα2 to modulate hepatic lipolysis and fatty acid metabolism
Correia JC, Massart J, de Boer JF, Porsmyr-Palmertz M, Martínez-Redondo V, Agudelo LZ, Sinha I, Meierhofer D, Ribeiro V, Björnholm M, Sauer S, Dahlman-Wright K, Zierath JR, Groen AK, Ruas JL
Mol Metab. 2015 Sep 26;4(12):891-902



